AUSTIN, Texas -- Following a Freedom of Information Act (FOIA) request from non-profit Informed Consent Action Network (ICAN), a previously undisclosed Merck study looking at the safety of Varivax vaccine revealed an alarming increase of adverse events in children who received the Chickenpox and MMR vaccine at the same time.
Moreover, the study, produced by the FDA Food & Drug Administration, revealed that the FDA and pharmaceutical corporation Merck were aware of these harms to children, yet failed and continue to fail to warn pediatricians and parents about the risks.
In 1995, following approval of the Merck chickenpox vaccine VARIVAX, the FDA required a post-licensure safety study which Merck conducted from June 1, 1995 through February 5, 1997. In 2019, ICAN, though its attorneys at Siri & Glimstad, filed a FOIA request for that study which the FDA produced a few months ago.
Merck's Phase IV post-licensure study included 34,655 children, 12-23 months old, and 51,463 children 2 to 12 years of age, all of whom were injected with VARIVAX. About 60% of the children aged 12-23 months and 17% of the children aged 2-12 years also received the MMR vaccine, and potentially other vaccines, at the same time they received VARIVAX.
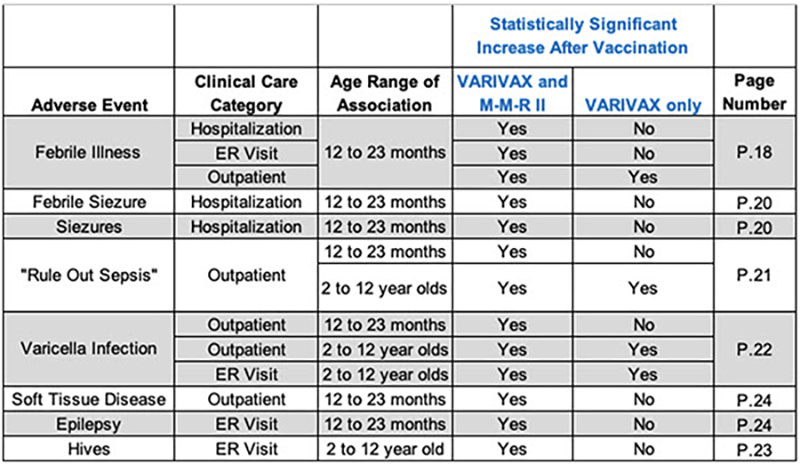
The study found several troubling safety signals: More than 60 conditions were significantly elevated following vaccination with both MMR and VARIVAX: Allergic reactions, alopecia, arthritis/arthralgia, gastroenteritis, among other alarms.
"The study strongly suggests this vaccine, soon after administration with MMR, leads to a wide range of reactions that in many cases are worse than the usual chickenpox rash," says ICAN Founder and CEO Del Bigtree. "What's even more alarming, however, is that this information has never been shared with the public."
The study shows that as early as September 1997, the FDA and Merck were both aware of the likelihood of the vaccines' combined danger to children but did nothing (See the accompanying chart).
"Once it had this information, what did FDA do about it?" Bigtree asks. "Did it reevaluate the VARIVAX licensure or guidance? Did it reevaluate the licensure or guidance of MMR or MMRV? Did it, at the very least, immediately recommend that VARIVAX and MMR no longer be given concomitantly? The answer to all three is 'No,' and that's still the answer, 25 years later."
More than two decades later, the FDA has not yet updated the package inserts for MMR to disclose potential harms. In fact, the package insert for VARIVAX explicitly claims that administering the two vaccines together is perfectly safe: "VARIVAX may be administered concomitantly with MMR."
"This is not the first time FDA has been caught hiding vaccines' damage to America's children from the nation's parents, as previous ICAN FOIA requests have proven," points out Bigtree. "Our prior FOIA proved the initial MMR vaccine trials had no placebo control and showed alarming increases in gastrointestinal illness and upper respiratory illness."
The Emmy-Award winning Bigtree currently hosts the breakout weekly investigative news program "The Highwire with Del Bigtree," which streams live weekly at 2pm EST/11am PST on Thursdays at thehighwire.com.